Is Everybody else rounding off data in their head? Or is she working with a unique thermometer? Notice that her numbers are constantly reduced than All people else’s readings… and the like.
Unique: Data ought to be in its original sort or maybe a Accredited genuine duplicate. Any alterations to the original data need to be traceable.
The viewpoints, information and conclusions contained in just this blog really should not be construed as conclusive fact, ValGenesis supplying assistance, nor as an indication of upcoming success.
By integrating these finest practices into their data administration procedures, corporations can foster a lifestyle of meticulous data governance, making certain adherence towards the ALCOA+ principles and bolstering their regulatory compliance endeavours.
Validating a form industry in essence signifies that the software checks you’re not earning any apparent mistakes or omissions. It warns you of Those people ahead of the data is submitted. Consider the last time you crammed out a signup sort on the net.
In any copy or transfer operation, make sure the suitable metadata is usually copied, and In case the metadata is actually a separate file object validate its integrity subsequently.
Accomplish an Assessment in the Uncooked data and metadata formats, audit trails and enter controls of Digital devices as aspect of their validation. Evaluate these in opposition to ALCOA+.
Attributability: This factor emphasizes the significance of figuring out the individual answerable for data entry or performing a selected motion, along with the time of this kind of functions.
This dictates that the collection time in the data really should correspond towards the date from the recording on the data.
The day column demonstrates not just inconsistencies in format, but in addition inconsistencies in day get. What took place at the end of website August? Was this another person taking place holiday, or was the fridge away from motion?
Applying demanding typical running treatments (SOPs), conducting standard audits, and employing automated units can help sustain both of those completeness and regularity. Businesses must also carry out periodic assessments to confirm that every one data is becoming managed In keeping with regulatory guidelines.
FDA together with other regulators see exactly the same difficulties pop up time and time once more. Several of these illustrations ended up taken from publicly offered FDA warning letters, but there is small question that EU regulators see the exact same difficulties. They often drop into 4 groups.
Saurabh Joshi ValGenesis delivers integrated and sensible remedies that assistance the electronic transformation of the lifetime sciences market. Which has a portfolio that covers The full solution lifecycle, ValGenesis provides a digital or specialized Alternative that brings worth to each stage of your validation and producing procedures as well as their similar functions.
These connected data ought to persist while in the archives to the lifetime of the history to allow them to keep on check here to assist the data if questions come up.
 Jennifer Grey Then & Now!
Jennifer Grey Then & Now!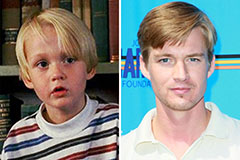 Mason Gamble Then & Now!
Mason Gamble Then & Now! Romeo Miller Then & Now!
Romeo Miller Then & Now!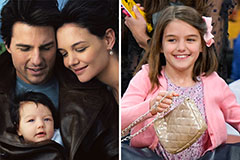 Suri Cruise Then & Now!
Suri Cruise Then & Now! Morgan Fairchild Then & Now!
Morgan Fairchild Then & Now!